Description
Arsine AsH3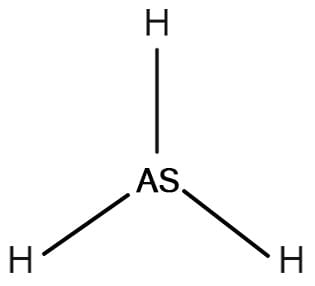
Arsine, or arsenic trihydride, is a non-organic compound of arsenic and hydrogen. At ambient temperature, it takes the form of a colourless, toxic and flammable liquefied gas. Arsine is heavier than air and smells like garlic or fish and is soluble in water. Today, arsine is now called “arsane” by the IUPAC.
Professional use of Arsine (AsH3)
Arsine is mainly used to produce circuits for the semi-conductors industry, as well as lead batteries and crystals for optical fibres and computer chips. It is also a doping agent used in the refinery and metals sector, or in chemistry for organic synthesis.
Risks of Arsine
Arsine is highly toxic to humans – it is the most toxic form of arsenic. Routes of exposure to arsine are inhalation and contact with skin and eyes. Due to its flammable and pyrophoric nature (it spontaneously ignites when exposed to air), arsine can also cause fires and explosions.
Effects on the human body
– In case of repeated exposure, arsine can cause symptoms such as dizziness and vertigo, lack of coordination, nausea, abdominal pain, vomiting, chest tightness and chest pain; prolonged exposure leads to asphyxia.
– The liquid vapours of arsine can cause burns and frostbite affecting the eyes and skin (even when wearing gloves). Ocular lesions can cause increased sensitivity to light, corneal scarring and/or blurred vision.

